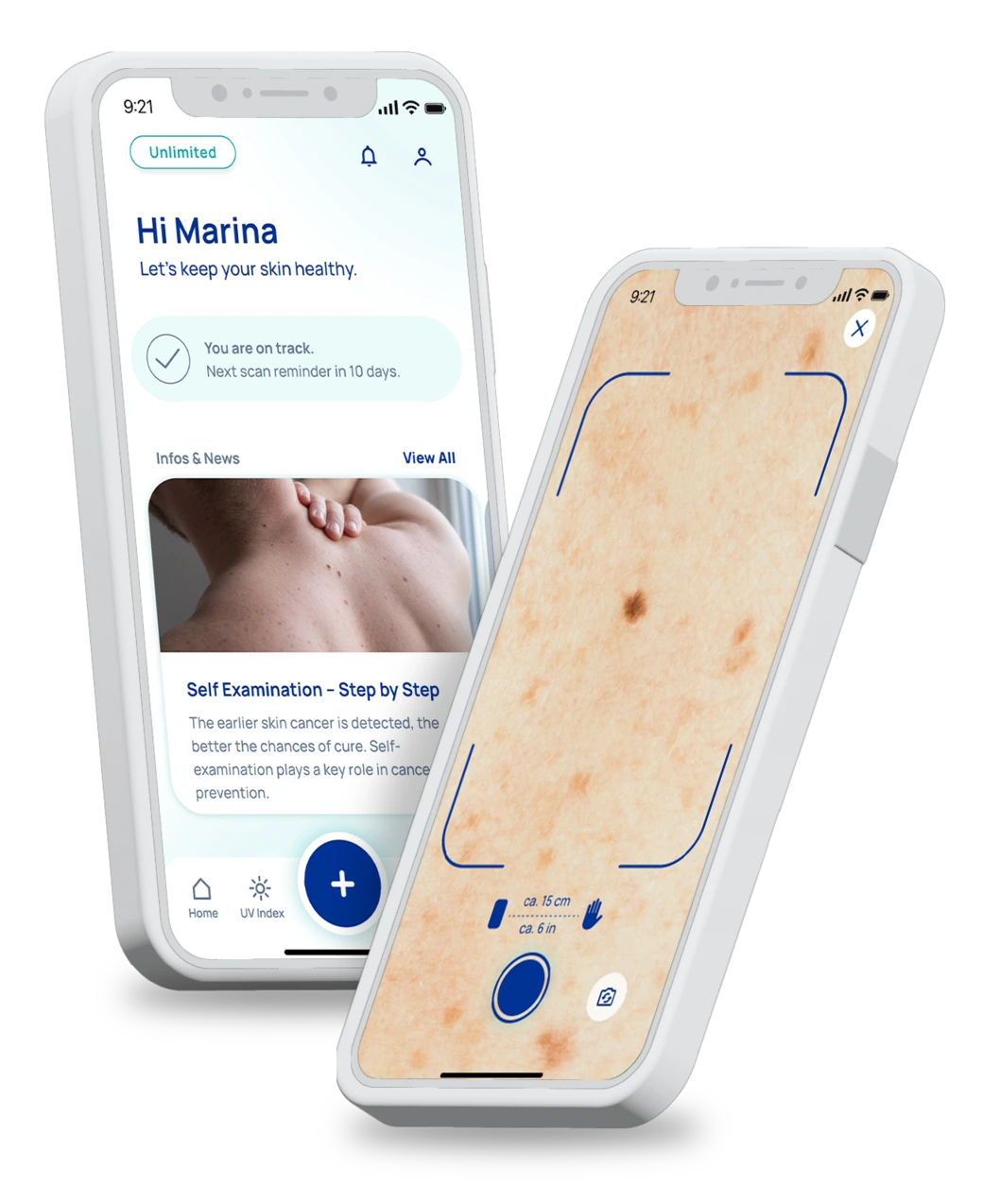
According to the EU Regulation 2017/745 (MDR) Chapter I, Article 2/1, a medical device is an instrument, apparatus, appliance, software, implant, reagent, material or other article which, according to the manufacturer, is intended for human use and which, alone or in combination, is intended to fulfill one or more of the following specific medical purposes: Diagnosis, prevention, monitoring, prediction, prognosis, treatment or alleviation of disease, diagnosis, monitoring, treatment, alleviation of or compensation for injury or disability, examination, replacement or modification of anatomy or of a physiologic or pathologic process or condition, obtaining information by the in vitro examination of specimens derived from the human body, including organ, blood and tissue donations, and whose principal intended action in or on the human body is not achieved by pharmacological, immunological or metabolic means, but whose mode of action may be enhanced by such means.